A team of scientists supported by the National Institutes of Health (NIH)’s The BRAIN Initiative®, including Davi Bock, Ph.D., Associate Professor of Neurological Sciences at UVM’s Robert Larner, M.D. College of Medicine, recently made a substantial advancement in neurobiological research by successfully mapping the entire brain of Drosophila melanogaster, more commonly known as the fruit fly.
50 largest neurons of the fly brain connectome (Image: Tyler Sloan and Amy Sterling for FlyWire, Princeton University, [Dorkenwald et al.,2024])
A team of scientists supported by the National Institutes of Health (NIH)’s The BRAIN Initiative®, including Davi Bock, Ph.D., Associate Professor of Neurological Sciences at UVM’s Robert Larner, M.D. College of Medicine, recently made a substantial advancement in neurobiological research by successfully mapping the entire brain of Drosophila melanogaster, more commonly known as the fruit fly.
The study, titled “Whole-brain annotation and multi-connectome cell typing of Drosophila,” recently published in Nature, established a “consensus cell type atlas,” or a comprehensive guide, for understanding the different types of cells in the fruit fly brain. The fruit fly’s brain contains around 130,000 neurons (a human’s brain contains 86 billion; mice, which often stand-in for humans in scientific research and testing, have 100 million neurons). The electron microscopy dataset underlying the whole-brain connectome (known as FAFB, or “Full Adult Fly Brain”) uses the detailed shapes of every neuron in the fly’s brain as well as all the synaptic connections between them to identify and catalogue all cell types in the brain. This complete map will help researchers to identify how different circuits work together to control behaviors like motor control, courtship, decision-making, memory, learning, and navigation.
“If we want to understand how the brain works, we need a mechanistic understanding of how all the neurons fit together and let you think,” remarked study co-lead Gregory Jefferis, Ph.D. “For most brains we have no idea how these networks function. Now for the fly we have this complete wiring diagram, a key step in understanding complex brain functions. In fact, using our data, shared online as we worked, other scientists have already started trying to simulate how the fly brain responds to the outside world.”
While similar studies have been done with simpler organisms, such as the nematode worm C. elegans and the larval stage of the fruit fly, the adult fruit fly offers more intricate behaviors to study. Though the fruit fly’s brain is clearly less complex than that of a human, or even a mouse, the implications of the study are profound. There are tremendous commonalities in how neural circuits in all species process information; this work allows principles of information processing to be identified in a simpler model organism and then sought in larger brains. Bock notes that scientists are currently incapable of scaling up this approach to a human brain, but states that this achievement represents a noteworthy step toward complete connectome of a mouse brain.
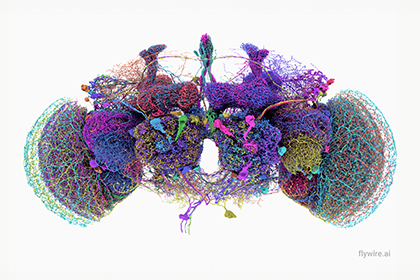
All 139,255 brain cells in the brain of an adult fruit fly. Activity within these cells drives an entire organism, from sensory perception to decision making and the control of actions, such as flying. (Credit: Tyler Sloan for FlyWire, Princeton University, [Dorkenwald et al.,2024])
“This type of work [being done across this field of connectomics] advances the state of the art in a once-in-a-century fashion, allowing us to both map the shapes and connections of every individual neuron in the complete brain of a fairly sophisticated animal, the adult fruit fly, and to annotate and mine the resulting connectome with cutting-edge software analytics. Neither light microscopy—even with multi-color fluorescence—nor the classical Golgi method and its allied approaches has provided this capability,” said Bock. “To achieve this feat at the scale of the entire brain of an important genetic model organism such as the fruit fly represents a remarkable advancement in the field.”
This study leverages tools and data generated by the FlyWire Consortium, which includes study leads such as UVM’s Bock; Gregory Jefferis, Ph.D., and Philipp Schlegel, Ph.D., from the MRC Laboratory of Molecular Biology and University of Cambridge; and Sebastian Seung, Ph.D. and Mala Murthy, Ph.D., of Princeton University. The consortium used electron microscopic brain images generated previously in Bock’s lab to create a detailed map of connections between neurons in the entire adult brain of a female fruit fly. This map includes around 50 million chemical synapses between the fly’s aforementioned 139,255 neurons. Researchers also added information about different types of cells, nerves, developmental lineages, and predictions about the neurotransmitters used by neurons. FlyWire’s Connectome Data Explorer open-access data analysis tool is accessible and available for download, and can be browsed interactively—all done in the spirit of encouraging team science. This work is detailed in an accompanying Nature paper, “Neuronal wiring diagram of an adult brain.”
“We have made the entire database open and freely available to all researchers. We hope this will be transformative for neuroscientists trying to better understand how a healthy brain works,” stated Murthy. “In the future we hope that it will be possible to compare what happens when things go wrong in our brains, for example in mental health conditions.”
By tracing connections from sensory cells to motor neurons, researchers can uncover potential circuit mechanisms that control behaviors in fruit flies, marking a crucial step toward understanding the complexities of human cognition and behavior.
“The diminutive fruit fly is surprisingly sophisticated and has long served as a powerful model for understanding the biological underpinnings of behavior,” said John Ngai, Ph.D., director of the study’s funding party, NIH’s The BRAIN Initiative®. “This milestone not only provides researchers a new set of tools for understanding how the circuits in the brain drive behavior, but importantly serves as a forerunner to ongoing BRAIN-funded efforts to map the connections of larger mammalian and human brains.”
The FlyWire Paper Package
The finished flywire connectome is reported by two papers, in which Dorkenwald et. Al. and Schlegel et. Al. jointly describe the resource:
The "flagship" paper, an overview of the connectome: Neuronal wiring diagram of an adult brain (Dorkenwald et. al.)
Annotations and cell typing: Whole-brain annotation and multi-connectome cell typing quantifies circuit stereotypy in Drosophila (Schlegel et. al.)
Seven additional papers enrich and analyze the connectome:
Visual system and cell types in the optic lobes: Neuronal “parts list” and wiring diagram for a visual system (Matsliah et. al.)
Connectome network analysis: Network Statistics of the Whole-Brain Connectome of Drosophila (Lin et. al.)
Virtual fly simulation: A Drosophila computational brain model reveals sensorimotor processing (Shiu et. al.)
Connectomic reconstruction predicts the functional organization of visual inputs to the navigation center of the Drosophila brain (Garner et. al.)
Neural circuit mechanisms for context specific halting in Drosophila (Sapkal et. al.)
The fly connectome reveals a path to the effectome (Pospisil et. al.)
Predicting visual function by interpreting a neuronal wiring diagram (Seung)